COVID-19: The T Cell Story
Now more than six months in, this has not happened, and it does not appear to be close to happening. A few months ago, noted virologist Christian Drosten mused in his (German) podcast that some people might have a level of immunity after all, but he wasn’t yet sure. Turns out he may have been right.
In this post, I hope to cast some light on:
- The slightly mysterious slowing down of the COVID-19 epidemic
- The several layers of our immune system
- Cell Mediated Immunity / T cells
- How around 50% of us appear to have (some?) Cell Mediated Immunity against COVID-19
- What this might mean (spoiler: not sure)
NOTE: Our immune system is astoundingly complex. Thousand page books call themselves “introductions”. For this page, I’ve sadly had to leave out many details, but the concepts described should provide a useful understanding. To read a more detailed description by an actual expert, I highly recommend you head to this page by Derek Lowe.
The scale of the epidemic
Although the human toll is staggering, this is not the case everywhere, nor is it as bad as was feared. Some countries have mounted impressive test & trace programs that clearly helped. Other regions have however not taken very drastic actions (or only belatedly) and somehow have not seen very large outbreaks (so far). And wherever we look, infections level off before 10%-20% of the population is infected (with some notable concentrated exceptions, like some villages in Italy reporting far higher or far lower numbers). This is somewhat mysterious.
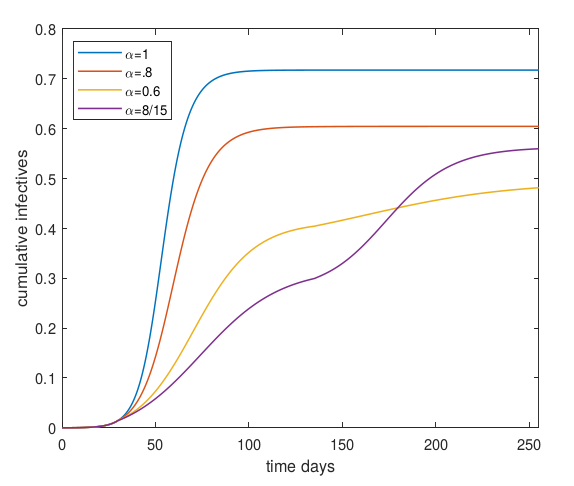
One model’s predicted fraction of population that will get infected. Alpha=1 stands for no control measures. Details in this post
NOTE: This article in no way argues for rapid lifting of control measures or lock-downs. If you read it that way, that is all up to you. I personally am still going (almost) nowhere.
Under many conditions, a COVID-19 patient will infect 2 or 3 additional people and simple models show that an epidemic should then spread rapidly until it has infected ~60% of the population - at that point so many people are immune that on average, less than one new person gets infected per case.
It has however also been observed that most people with COVID-19 go on to infect nobody, often not even people living in the same house or sleeping in the same bed (!).
This phenomenon is called overdispersion, and it means that while on average a patient infects 2 or 3 new people, this average consists of many people infecting nobody, and then some mass spreading events infecting many more.
When epidemics are modeled taking such variable susceptibility and spreading into account, it is found that an epidemic might level off (way) before 60% of the population is infected.
This concept is explained in my earlier post “R and the Herd: Why we could be nearer to community immunity than we think”. Very briefly, if it turns out that a significant fraction of the population is far less susceptible to infection, the virus needs to do its spreading through of a rapidly dwindling pool of candidates. Or in other words, if we assume (we have no data) that only 15% of the population is easily infected, soon enough that 15% actually is infected, leaving the virus with an uphill battle to spread through people that are hard to infect.
“The dose makes the poison” - in vaccine trials, animals are exposed to staggering doses of virus, just to make sure the control group (without a vaccine) actually gets infected. There is also the concept of ‘minimum infective dose’. It may be that for some people the MID is way higher than for others. A very low vitamin D status might make the dose lower.
Very serious scientists think the idea of variable susceptibility (& its impact on how many people will get infected) might have legs, but we do need to explain why some people are far more easily infected than others.
And here we enter the vast complexity of our immune system.
Recall that originally it was found that no one carried antibodies that worked on COVID-19, but here I claim that some people might have some functioning immunity. How can this be?
The many layers of our immune system
Our immune system has multiple layers. For starters, there is physical protection, where mucus can prevent a virus particle from coming into contact with a living cell in the first place. Secondly, our “innate” immune system is capable of defeating many potential infections, even from unknown pathogens. This is our “standing army”.
If we are lucky, or if the dose of the virus is low enough, these initial layers might be enough to protect us from becoming (seriously) ill.
The most potent force of our immune system is the “adaptive” part. Over the course of days or weeks, this forms the famous antibodies that stick to virus particles, thereby disabling them directly, or flagging the virus for destruction.
The process by which our bodies evolve antibodies is utterly fascinating, and I wrote about this in an earlier post. Prepare to have your mind blown.
Discovering if people have antibodies is relatively simple, especially if you have a PhD in molecular biology and spent a decade training in a lab. Through very sensitive surveys, it is possible to sniff out antibody activity against viral components. This is the kind of test that led to the fear there was no immunity against SARS-CoV-2.
SARS-CoV-2 ‘Spike’ protein, by 5-HT2AR
From all the noise made around antibodies, one could be forgiven for thinking they are the entire adaptive immune system. Nothing however is farther from the truth.
Stemming the source
As explained in my earlier post, viruses can only multiply with our help. They hitch a ride on the molecular machinery in our cells - we are ourselves the factories of what makes us ill.
Antibodies are pretty miraculous in that they can disable virus particles with great efficiency.
But clearly, as long as our bodies keep churning out trillions of new copies of a virus, this is not the complete solution.
Enter “cell mediated immunity”. Cell mediated immunity gets rid of cells that are infected and perform replication of the disease. By disabling (killing) infected cells, the reproduction of the virus can be halted.
So in short, antibodies disable existing copies of the virus, while cell mediated immunity attempts to stop the generation of new virus particles.
Actually, cell mediated immunity does many more things - it mediates antibodies as well, plus much much more. Derek Lowe, one of the COVID-19 explainers-in-chief, goes into some depth in this article, in which he laments after nearly a thousand words: “And you’re going to hate me for saying this, but that’s where things get complicated.”
How it works
How do you figure out if a cell is infected? Virus particles are exceptionally tiny, and we can’t put a camera on every cell to see what is going on.
Nature has come up with a stupendously powerful solution. When a cell does things, it creates proteins from DNA. Originally it was thought every one of our 20000 genes was responsible for one kind of protein, but we now know around 1 million types of proteins are in common use by our bodies.
Earlier, I wrote a reasonable compact read called “What is life” which explains the relation between DNA, RNA, amino-acids and proteins.
Proteins are used by cells to do things, but after a while they also degrade, or are scaffolding left over from the process of building bigger proteins. Every cell therefore has a lot of protein parts floating around.
Snippets of these proteins are collected, mounted on a special Major Histocompatibility Complex (MHC) molecule, and transported to the surface of a cell. The way to imagine this is that every cell is covered with MHC molecules, each presenting a bit of protein to the immune system for inspection.
A large but still countable number of different snippets (aka peptides) are presented by our cells, a number that is limited enough that our immune system can learn about all the good peptides, also known as the “self peptides”.
In our blood and lymph system circulate cells that recognize the peptides presented on the MHC molecules, and these cells have been evolved specifically to leave the self peptides alone.
The moment a cell becomes infected, it gets co-opted by the virus to create proteins that form new virus particles. Eventually, snippets (peptides) from those viral proteins are picked up, joined with an MHC molecule, and then presented on the outside of the cell.
T cell, picture source: NIAD
This is in effect a cell waving a flag “I am infected!”, and the immune system picks up on that. In the case where a similar infection has been seen earlier, “T cells” are already available that will recognize the strange new peptide. These T cells then do many things, which eventually leads to a “killer T-cell” to move in to rid the body of the infected cell.
Now, viruses have been around for a while already, and they attempt to shut down the whole MHC presenting process so that they might evade discovery. But our immune system has also been perfecting its skills for 100s of millions of years.
If a cell is no longer presenting sufficient “good” peptides on its surface, natural killer cells move in to destroy it. This is like a negative control.
So to summarise, to survive, a cell must continuously present “good” peptides mounted on MHC molecules. If this “good” signal fades, the cell gets killed. If non-self peptides are presented, the cell gets killed. So in short, the cell has to work very hard to stay alive
What has been found
As noted, doing antibody studies is “relatively easy”, although it takes work to do it really well. To detect antibodies against COVID-19, “all we have to do” is recreate some of the outer parts of the virus particle, expose these to blood, and see if something sticks.
To recreate these parts, we insert genes from the virus into bacteria which then go on to use their cellular machinery to create proteins for us. It never ceases to amaze me that the code of life is so universal we can get bacteria to “run” DNA meant for humans!
So, relatively quickly, we were able to do antibody tests on various populations, and the news was disappointing - nothing reacted.
But what about the cell mediated immunity? Turns out this is way harder. T cells connect to protein snippets presented on MHC molecules on cell surfaces. So to do a naive test, we should convince a cell to mount a piece of COVID-19 on an MHC molecule on its surface, and then see if any T cells want to stick on it. This is not practicable.
In reality, using computer models, we try to predict what kind of peptides an infected cell would show. We then create a pool of all those peptides. Then from blood we are interested in, specific cells (PBMC) are isolated and these are mixed with the pool. If relevant (reactive) T cells were around, this can be detected indirectly with some efficiency - but it is by far not as precise as the ELISA antibody tests.
A handful of separate groups have now done the hard work to learn about T cells and COVID-19, and the news is generally good - our bodies create robust T-cell (and B-cell) responses to an infection (see links at end of the article).
But somewhat surprisingly, around 40%-50% people never exposed to COVID-19 do appear to have a measurable T-cell reaction against the new virus.
This may be due to the four major other Coronaviruses currently circulating, most of which are currently associated with simple colds. One of these is reasonably suspected to have been behind the “Russian Flu” of 1889-1890.
Now, how could it be that there are T cells but no antibodies for a virus? A big difference is that antibodies are only generated against the actual virus in its circulating form. The inner parts are not “seen” by the B-cells that make antibodies. In addition, viruses make more proteins than end up in the final result. Some proteins are “non-structural” and are only used during assembly, for example.
It may well be that various of these “inner workings” are more stable across the Coronavirus family than the outer parts.
So what does it mean?
Does this mean that if you recently had a Coronavirus that caused a head cold that you have a measure of protection? Or perhaps that the course of the disease will be milder?
And might some regions of the world be having an easier time because the specific Coronavirus was circulating there in the months before?
Also, could the partial immunity be behind the “overdispersion” of COVID-19?
To be honest, we just don’t know. T-cell research is very hard work. There are some signs that COVID-19 is good at evading cell mediated immunity responses.
Some encouragement may however be found in the somewhat “disappointing” H1N1 influenza epidemic of 2009, where according to this paper, cross reactive T cells from other flu variants blunted the disease significantly.
We can be sure however that this subject will be studied intensively in the coming months. And who knows, the insights gained may help immunization or treatment efforts.
Relevant links, preprints and papers
My own articles:
- Vitamin D: the New COVID-19 Chloroquine?
- COVID-19 Predictions & Rampant Speculation: May 2020
- R and the Herd: Why we could be nearer to community immunity than we think
- Why no breakthrough yet? Or, why is it ’taking so long’ to test COVID-19 treatments?
- Our amazing immune system
Scientific papers, preprints, news articles:
- Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals
- SARS-CoV-2 epitopes are recognized by a public and diverse repertoire of human T-cell receptors
- Presence of SARS-CoV-2 reactive T cells in COVID-19 patients and healthy donors
- MHC hiding
- T cells may help COVID-19 patients — and people never exposed to the virus
